Iron 26Fe55.847
Known to ancient civilizations.
French: fer
German: Eisen
Italian: ferro
Spanish: hierro
Description: Iron, when absolutely pure, is lustrous, silvery and soft (workable). This is the most important of all the metals and it is used chiefly as steel in which there is carbon (up to 1.7%). Stainless steels are alloys with other metals, mainly nickel. Iron rusts in damp air and dissolves readily in dilute acids. Its uses are legion.
Iron single crystal properties
| State: |
single crystal |
|---|
| Crystal structure: |
bcc |
|---|
| Production method: |
Strain annealing |
|---|
| Standard size: |
diameter 6-10mm
thickness 1-2mm |
|---|
| Orientation: |
(100), (110) and (111) |
|---|
| Orientation accuracy: |
<2°, <1°, <0.4° or <0.1° |
|---|
| Polishing: |
as cut, one or two sides polished |
|---|
| Roughness of surface: |
<0.03µm |
|---|
| Purity: |
99.98% |
|---|
| Typical analysis (ppm): |
C 3
H < 1
O 9
N < 5
Cu 1.60
Fe 1.80
Ni < 1
Pb 0.30
Si 0.30
Ga, Hf and Ta are below the detection limit
|
|---|
Materials properties
| Density: |
7.86 g/cm3 |
|---|
| Melting point: |
1534.85 °C / 1808 °K |
|---|
| Boiling point: |
2749.85 °C / 3023 °K |
|---|
| Molar volume: |
7.09 cm3 |
|---|
| Thermal conductivity: |
80.2 [300 K] Wm-1K-1 |
|---|
| Coefficient of linear thermal expansion: |
12.3 x 10-6 K-1 |
|---|
| Electrical resistivity: |
9.71x 10-8 [293 K] Wm |
|---|
| Mass magnetic susceptibility: |
ferromagnetic |
|---|
| Young's modulus: |
152.3 GPa (cast); 208 GPa (steel) |
|---|
| Rigidity modulus: |
60.0 GPa (cast); 81 GPa (steel) |
|---|
| Bulk modulus: |
109.5 GPa (cast); 160 GPa (steel) |
|---|
| Poisson's ratio: |
0.27 (cast); 0.27 (steel) |
|---|
| Radii: |
Fe3+ 67; Fe2+ 82; atomic 124; covalent 116; van de |
|---|
| Electronegativity: |
1.83 (Pauling); 1.64 (Allred); 4.06 eV (absolute) |
|---|
| Effective nuclear charge: |
3.75 (Slater); 5.43 (Clementi); 7.40 (Froese-Fischer) |
|---|
| Number of Isotopes (incl. nuclear isomers): |
16 |
|---|
| Issotope mass range: |
49 -> 63 |
|---|
| Crystal structure, (cell dimentions / pm), space group |
bcc |
|---|
| X-ray diffraction: mass absorption coefficients: |
CuKα 308 (µ/r) / cm2g-1
MoKα 38.5 (µ/r) / cm2g-1 |
|---|
| Neutron scattering length: |
0.954 b/10-12 cm |
|---|
| Thermal neutron capture cross-section: |
2.56 sa / barns |
|---|
Biological data
| Biological role: |
Essential to all species |
|---|
| Toxicity |
|
|---|
| Toxic intake: |
200 mg, Iron (II) compounds are more toxic than iron (III) |
|---|
| Lethal intake: |
7 - 35 g |
|---|
| Hazards: |
Iron dust poses a moderate fir or explosion hazad: chronic exposure causes iron pneumoconiosis (welder lung). Iron deficiency leads to anaemia, but excess iron in the body causes liver and kidney dama |
|---|
| Level in humans |
|
|---|
| Blood: |
447 mg dm-3 |
|---|
| Bone: |
3 - 380 p.p.m. |
|---|
| Liver: |
250 - 1400 p.p.m. |
|---|
| Muscle: |
180 p.p.m. |
|---|
| Daily dietary intake: |
6 - 40 mg |
|---|
| Total mass of element in average [70 kg] person: |
4.2 mg |
|---|
Geological data
| Mineral | Formula | Density | Hardness | Crystal apperance |
|---|
| Goethite |
a-FeO(OH) |
4.28 |
5 - 5.5 |
orth., met. earthy brown |
|---|
| Hematite |
Fe2O3 |
5.26 |
5 - 6 |
rhom., met. earth grey |
|---|
| Lepidocrocite |
g-FeO(OH) |
4.09 |
5 |
orth., met. reddish-brown |
|---|
| Magnetite |
Fe3O4 |
5.175 |
5.5 - 6.5 |
cub., met. black |
|---|
| Siderite |
FeCO3 |
3.96 |
4 |
rhom., vit. yellow-brown |
|---|
| Chief ore: |
hematite, magnetite, goethite, lepididocrocite, siderite |
|---|
| World production: |
7.16 x 108 tonnes/year |
|---|
| Main mining areas: |
USA, Canada, Sweden, South Africa, Russia, India, Japan |
|---|
| Reserves: |
1.1 x 1011 tonnes |
|---|
| Specimen: |
available as chips, filings, foil, granules, and wire. Safe. |
|---|
| Abundances |
|
|---|
| Sun: |
3.16 x 107 (relative to H = 1 x 1012) |
|---|
| Earth's crust: |
41000 p.p.m. |
|---|
| Seawater: |
|
|---|
| Residence time: |
|
|---|
| Classification: |
recycled |
|---|
| Oxidation state: |
III |
|---|
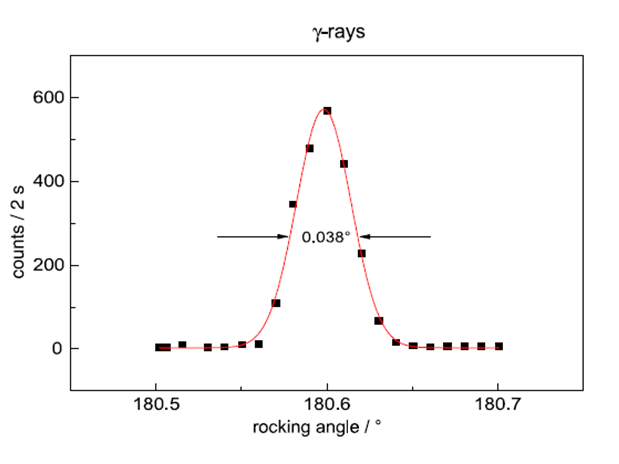
Measured mosaicity of Iron Single Crystal with Gamma diffractometry
Source: Emsley, J. (1998) The Elements (3rd Edition)

 English
English
 Deutsch
Deutsch

























































































